Gmp Product Quality Review The main objective of an annual product quality review is to see if your product consistently maintains its expected quality and to identify any needed improvements to ensure
The European Medicines Agency s EMA provides answers to frequently asked questions on good manufacturing practice GMP and good distribution practice GDP as discussed and Annual Product Quality Review is a mandatory requirement of Good Manufacturing Practice FDA uses the term Annual Product Review APR while Product Quality Review PQR term is
Gmp Product Quality Review

Gmp Product Quality Review
https://image2.slideserve.com/4591578/pic-s-gmp-product-quality-review-system-n.jpg

PPT PIC S GMP Product Quality Review System
https://image2.slideserve.com/4591578/iso-90002-n.jpg

PPT PIC S GMP Product Quality Review System
https://image2.slideserve.com/4591578/product-quality-review-pic-s-gmp1-5-l.jpg
The SOP for annual product quality review should details how to collect analyse and report data and information to provide a complete picture on product quality any Product Quality Reviews have become an accepted part of GMP requirements internationally and can provide useful information and additional controls over manufacturing processes and
Annual Product Quality Review APQR is an evaluation which is prepared according to the CGMP requirements of different regulatory authorities A Good Manufacturing Practice Guidance for Industry Product Quality Reviews Page 3 1 Introduction This guideline is intended to provide general guidance on the interpretation of the PIC S Guide to Good Manufacturing
More picture related to Gmp Product Quality Review

PPT PIC S GMP Product Quality Review System
https://image2.slideserve.com/4591578/quality-assurance-pic-s-gmp-l.jpg
Annual Product Quality Review APQR Format PDF Packaging And
https://imgv2-1-f.scribdassets.com/img/document/564200094/original/f6128cc846/1702608894?v=1

Annual Product Quality Review Software AmpleLogic YouTube
https://i.ytimg.com/vi/vuMLeE4FHq0/maxresdefault.jpg
This document provides guidance for the interpretation of the principles and guidelines of good manufacturing practice GMP for medicinal products as laid down in Directive 2003 94 EC for Preparing a comprehensive and compliant APQR report can be a complex task especially for pharmaceutical manufacturers operating under strict guidelines such as FDA
[desc-10] [desc-11]

Introduction To GMP Risk Assessment YouTube
https://i.ytimg.com/vi/PNmvFnyJ-Lw/maxresdefault.jpg

What GMP Documents Should Your QMS Include YouTube
https://i.ytimg.com/vi/kK3Id63-MtY/maxresdefault.jpg

https://www.gmpsop.com › annual-product-quality...
The main objective of an annual product quality review is to see if your product consistently maintains its expected quality and to identify any needed improvements to ensure

https://www.ema.europa.eu › en › human-regulatory...
The European Medicines Agency s EMA provides answers to frequently asked questions on good manufacturing practice GMP and good distribution practice GDP as discussed and

What Are GMP Guidelines Good Manufacturing Practices For Food Safety

Introduction To GMP Risk Assessment YouTube

Components Of GMP GMP In Pharmaceuticals Different Parts Of GMP
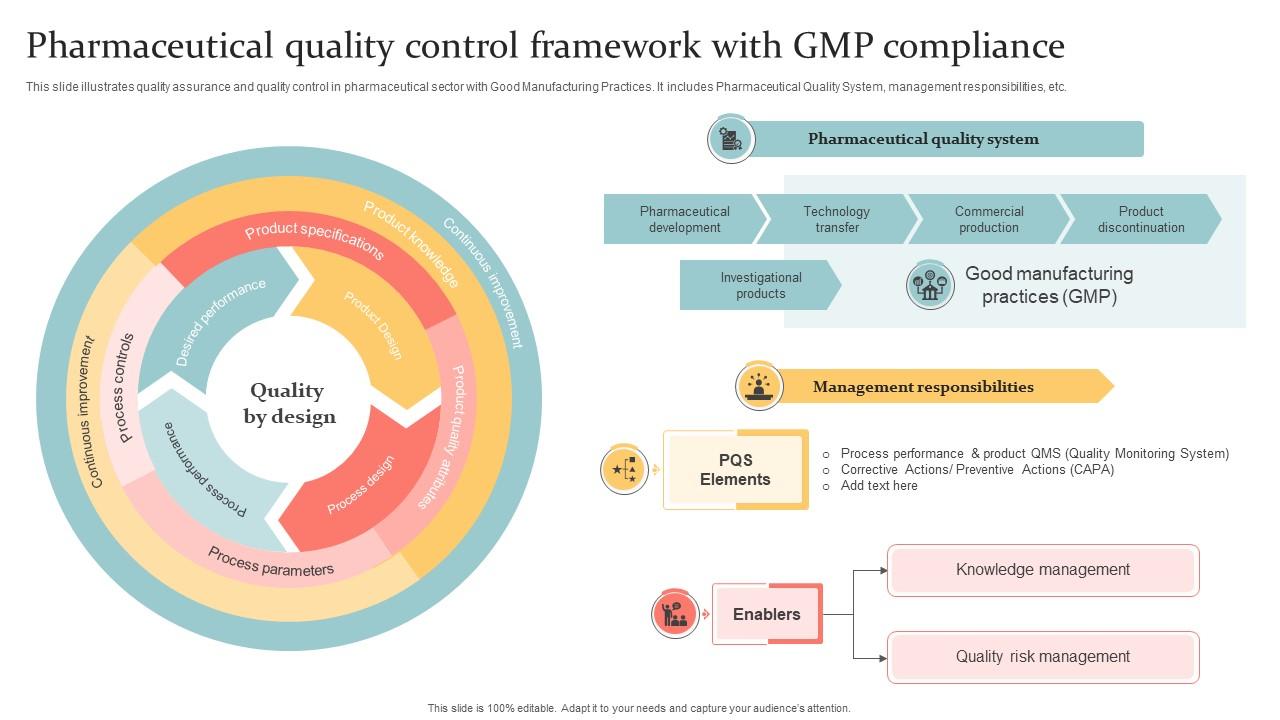
Pharmaceutical Quality Control Framework With GMP 48 OFF

2017 Pharmaceuticals Quality Assurance Validation Procedures GMPSOP
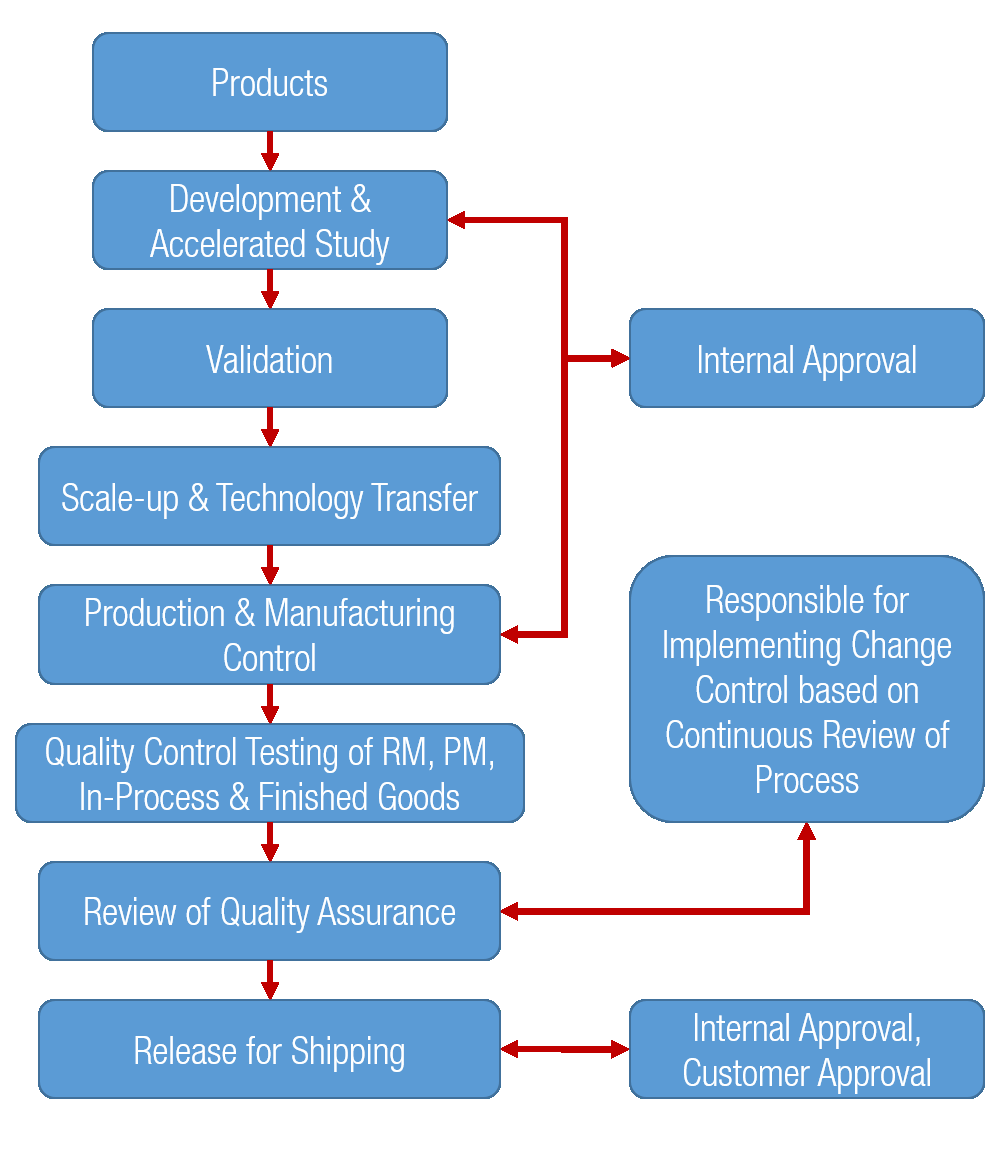
Mkppl gmp fc Murli Krishna Pharma

Mkppl gmp fc Murli Krishna Pharma
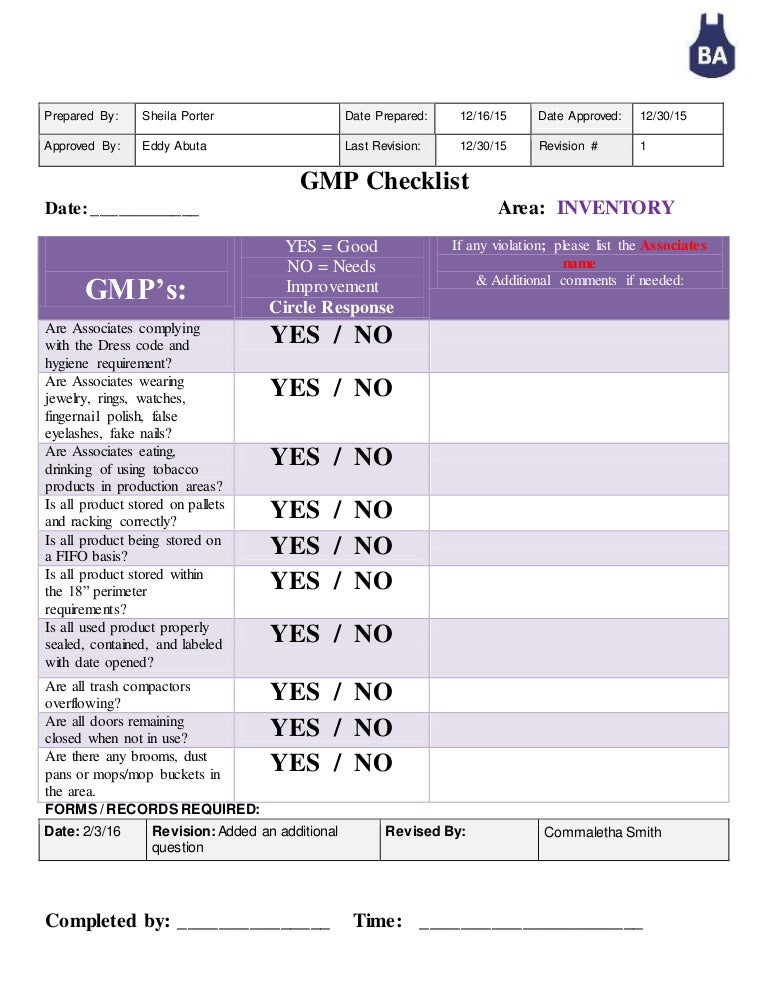
GMP Checklist INVENTORY

GMP Good Manufacturing Practice 6 Heading Of Infographic Template With

Johanna Wilhelmi Quality Assurance Manager Nycomed GmbH Takeda
Gmp Product Quality Review - [desc-14]
