Ich Gcp Definition Of Adverse Event The European Medicines Agency publishes scientific guidelines on human medicines that are harmonised by the International Council for Harmonisation of Technical Requirements for
1 About ICH 02 Purpose 03 Sphere of influence The International Council for Harmo nisation of Technical Requirements for Pharmaceuticals for Human Use ICH is unique in bringing Implementation of the ICH guidelines supports the alignment of regulatory requirements across regions reducing duplication of efforts and promoting consistent
Ich Gcp Definition Of Adverse Event
Ich Gcp Definition Of Adverse Event
https://i.ytimg.com/vi/Qntov1vVS-4/maxresdefault.jpg

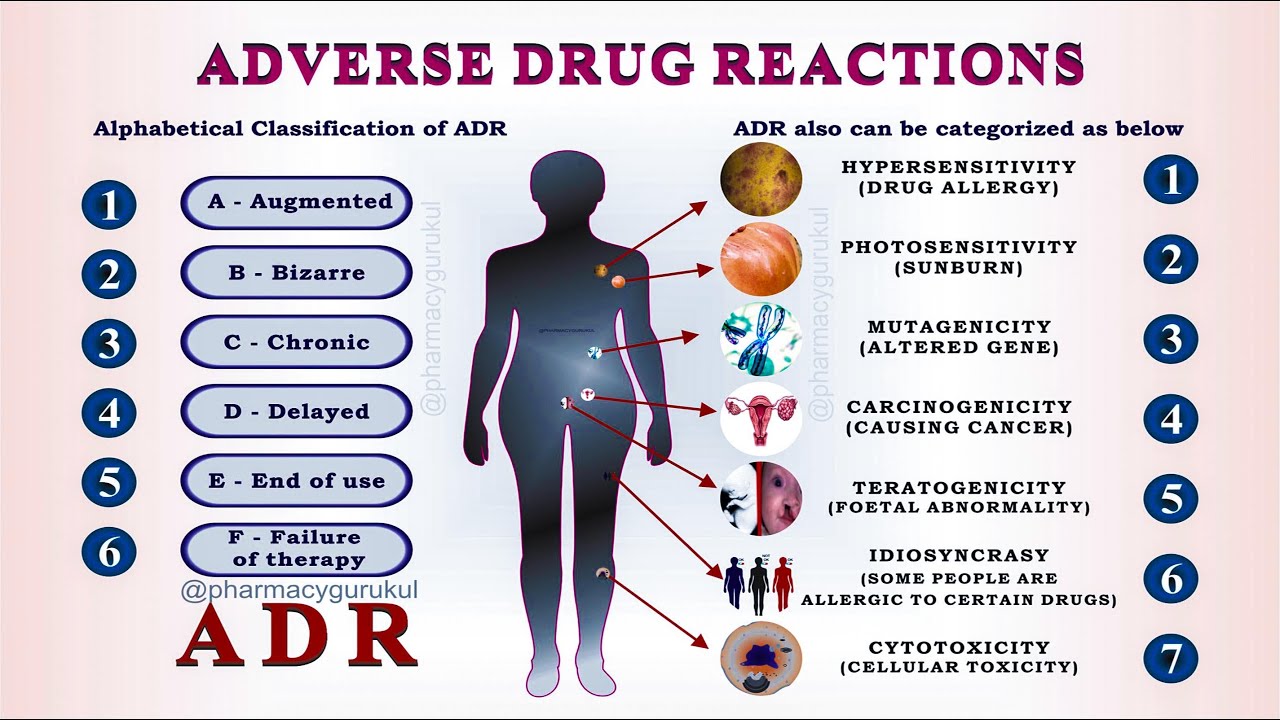
13 Principles Of ICH GCP Good Clinical Practices Guidelines In
https://i.ytimg.com/vi/TCW55krWzb4/maxresdefault.jpg

Vesicovaginal Fistula Wikipedia 48 OFF Brunofuga adv br
https://ichgcp.net/storage/images/ichgcp_plot_1200.jpg
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH is an initiative that brings together regulatory authorities and pharmaceutical The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH brings together the medicines regulatory authorities
The ICH topics are divided into the four categories below and ICH topic codes are assigned according to these categories The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH is unique in bringing together the regulatory authorities and pharmaceutical
More picture related to Ich Gcp Definition Of Adverse Event
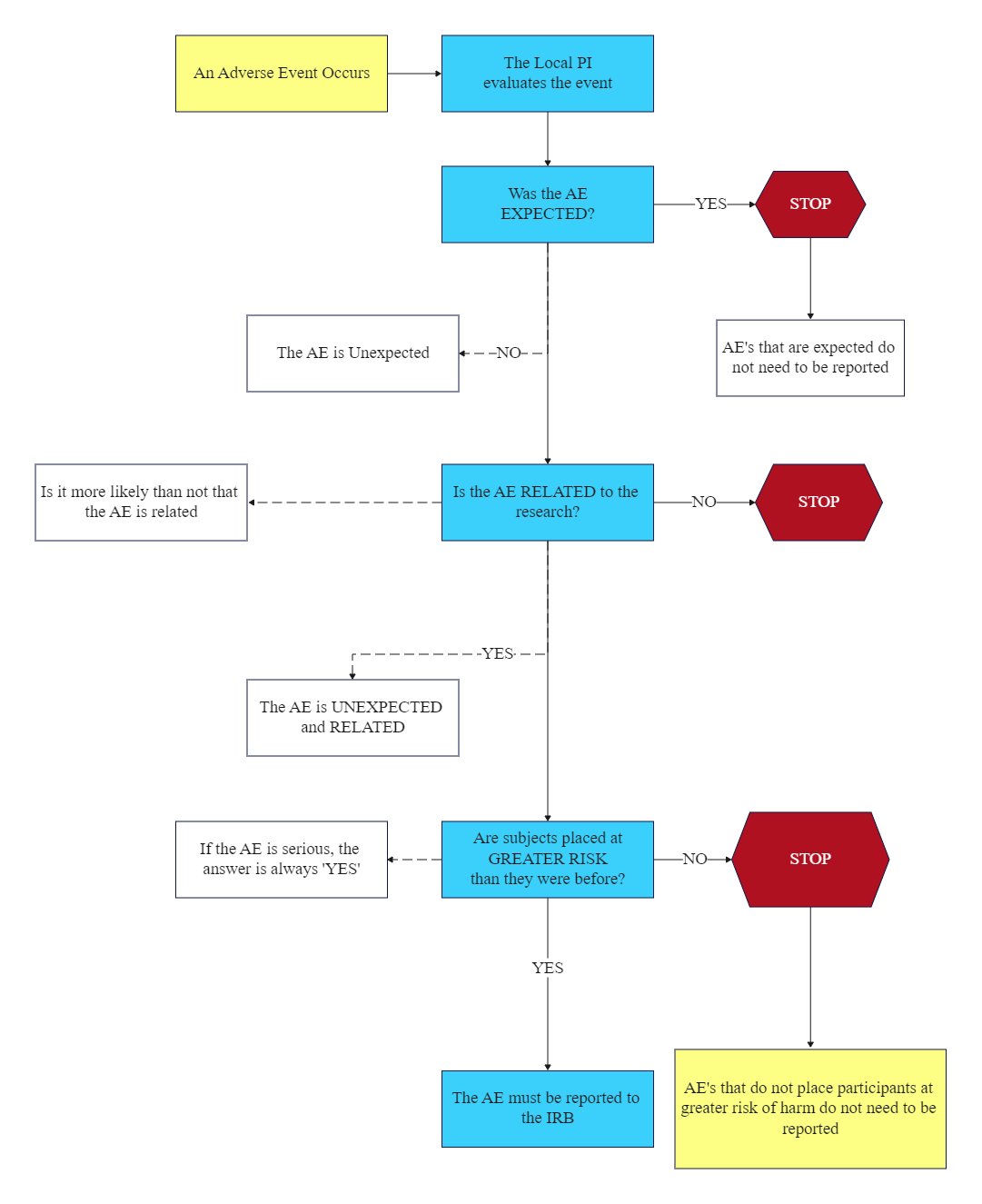
Adverse Event Flow Chart EdrawMax Template
https://edrawcloudpublicus.s3.amazonaws.com/work/1905656/2022-9-20/1663664784/main.png
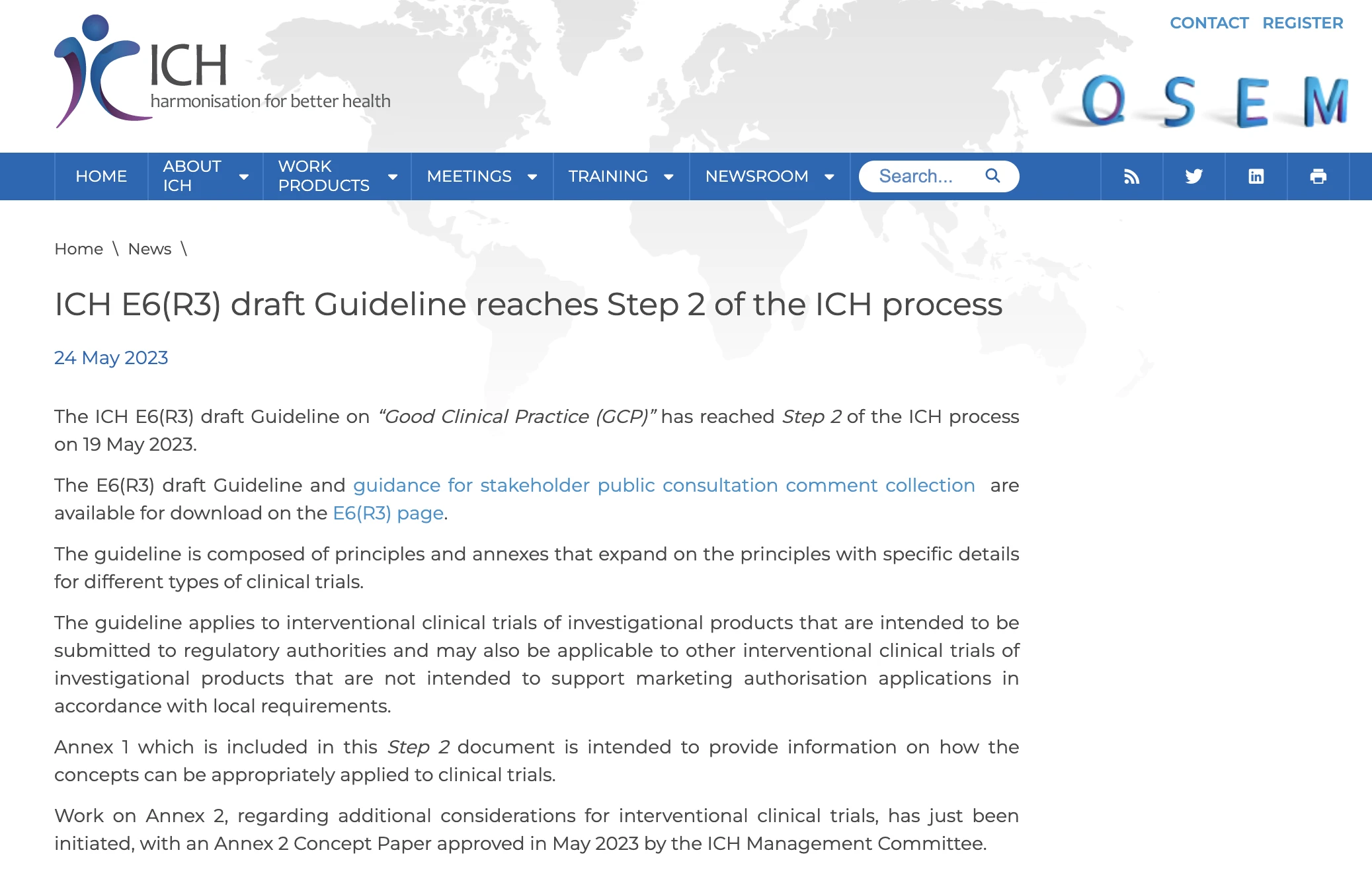
ICH E6 R3 ICH 2
https://i0.hdslb.com/bfs/article/watermark/ad3f08146c219db2cfdee369a8f068c4efb5a811.png
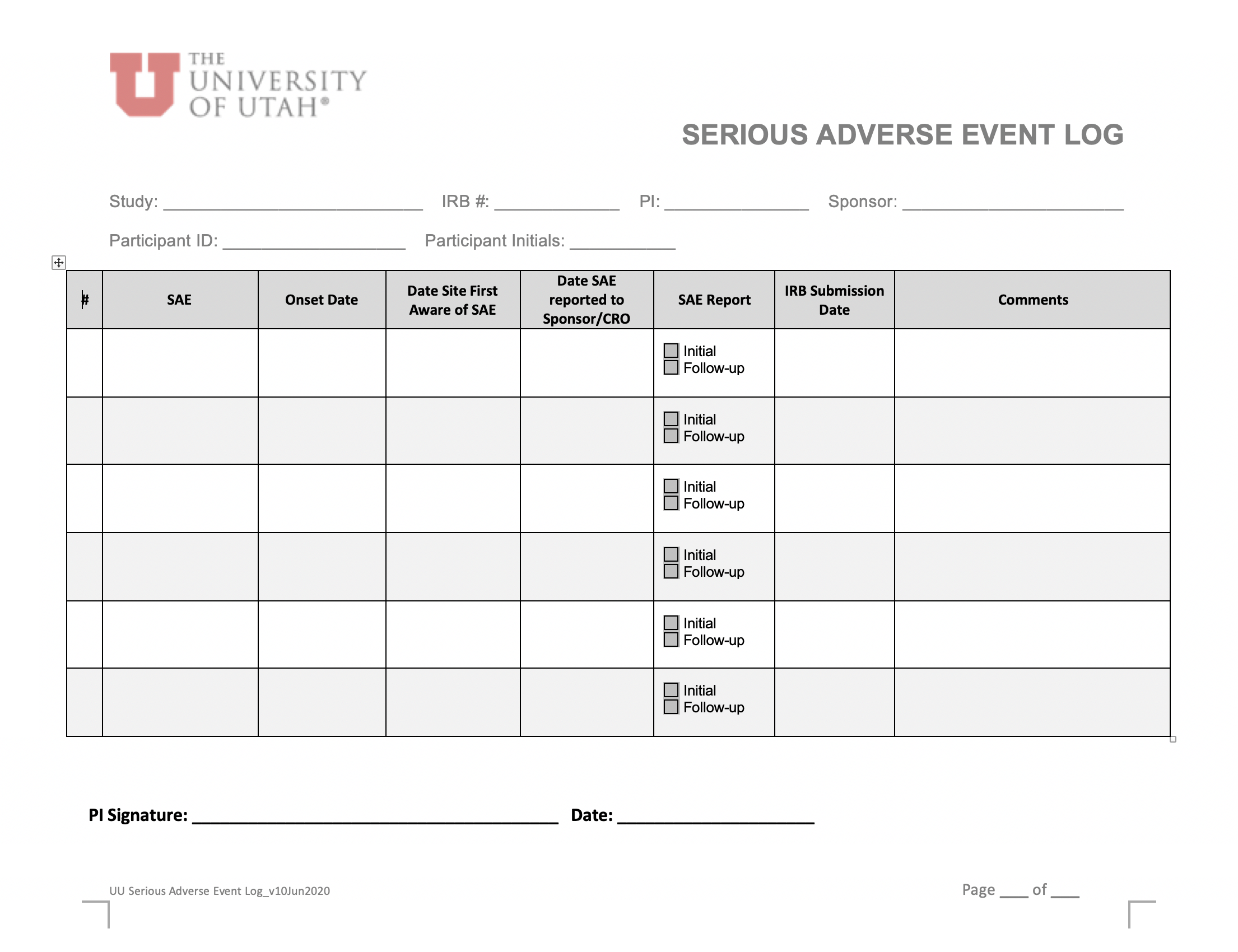
Tool Kit Research Quality And Compliance The University Of Utah
https://qualitycompliance.research.utah.edu/_resources/images/toolbox/serious-adverse-event-log.png
The ICH guideline for good clinical practice GCP establishes an international standard for the design conduct recording and reporting of clinical trials involving human The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the influence of a variety of environmental
[desc-10] [desc-11]

Event Reporting
https://uha.blob.core.windows.net/accelerate/attachments/cjvpqgbxz06af0gpc3cviznrl-safety-events-01.full.png

CELL DIVISION Mitosis Meiosis Ppt Download
https://slideplayer.com/slide/13089330/79/images/14/Metaphase+Bivalent+chromosomes+line+up+along+equator+of+cell+Chromosomes+attach+to+spindle+fibers.jpg

https://www.ema.europa.eu › en › human-regulatory...
The European Medicines Agency publishes scientific guidelines on human medicines that are harmonised by the International Council for Harmonisation of Technical Requirements for

https://admin.ich.org › sites › default › files
1 About ICH 02 Purpose 03 Sphere of influence The International Council for Harmo nisation of Technical Requirements for Pharmaceuticals for Human Use ICH is unique in bringing
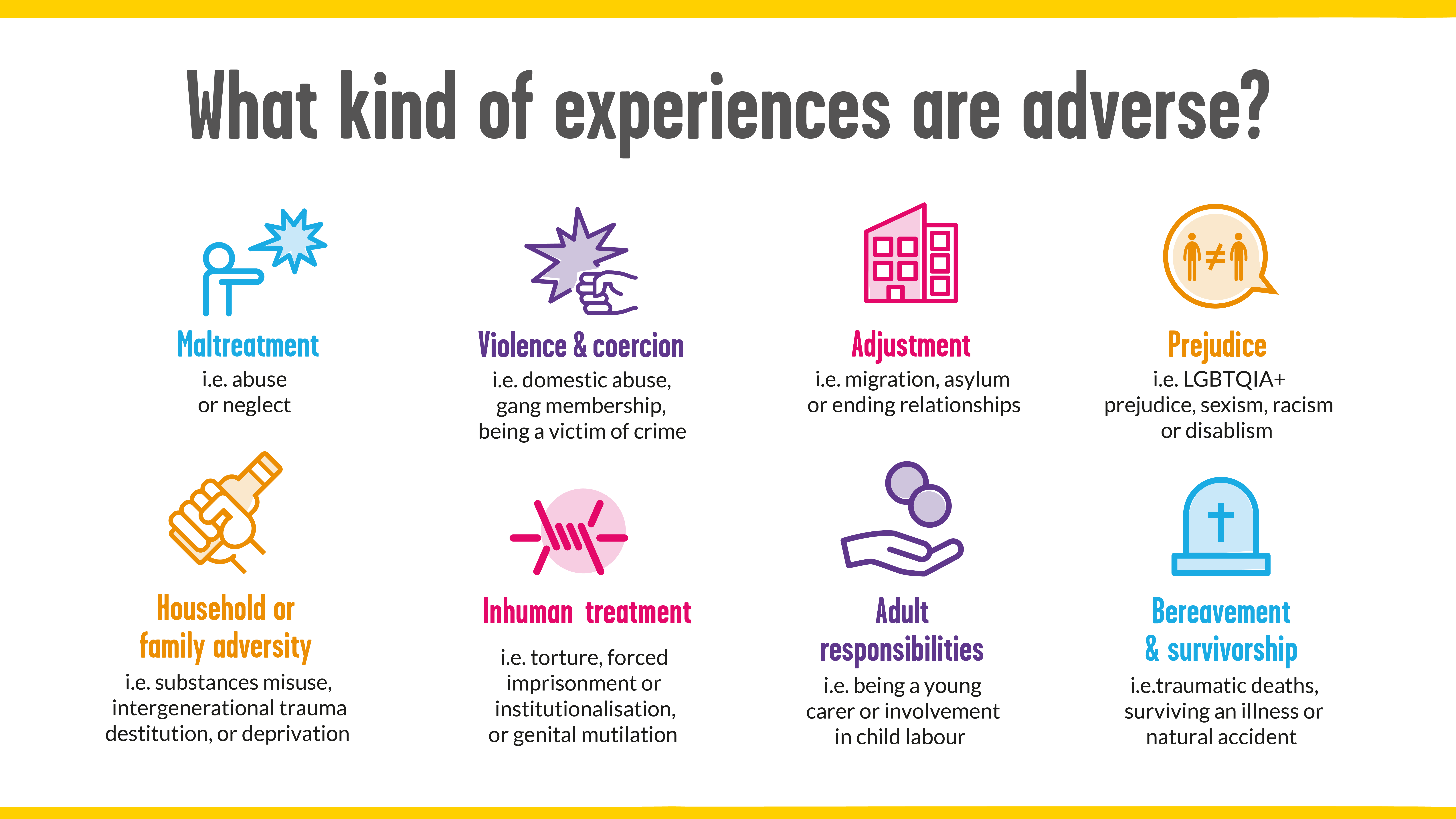
Understanding Trauma Vrogue co

Event Reporting
Summary
Diagram 1 Demonstrates The Reporting Process For External Adverse

Acu rite Digital 2 Axis Mill Pakage Kit For Bridgeports

Reporting Events Telegraph

Reporting Events Telegraph

Comparison Tool ICH E6 R3 Draft To ICH E6 R2 Clinical Pathways

CCRB ICH GCP Knowledge Test
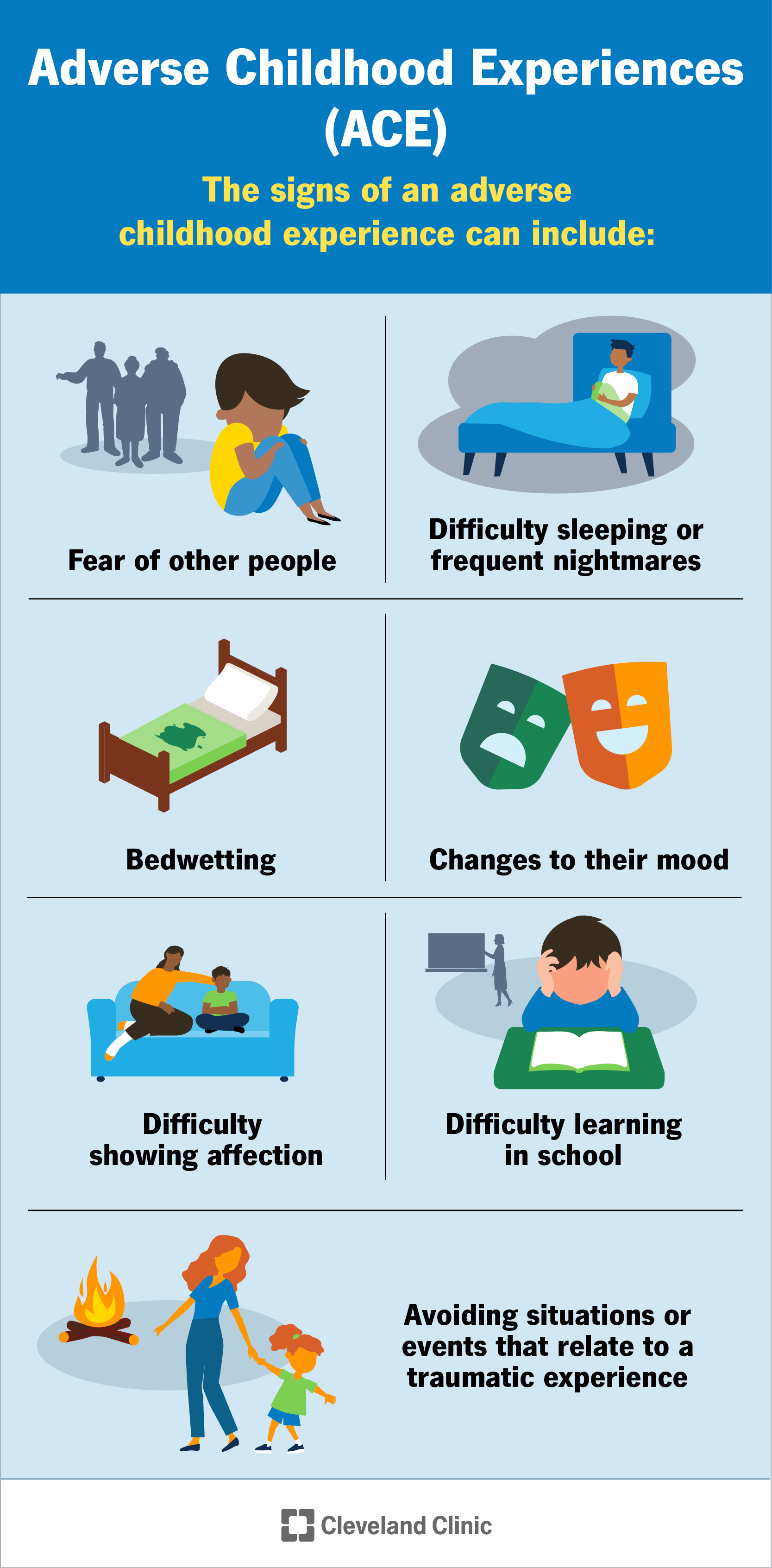
Adverse Childhood Experiences
Ich Gcp Definition Of Adverse Event - [desc-12]