Ich Gcp Full Form 3 3 Stability Indicating Critical Quality Attributes g the principles outlined in ICH Q6A Q6B and ICH Q8 Q11 When 331 designing a stability protocol in support of a drug substance or drug
The European Medicines Agency publishes scientific guidelines on human medicines that are harmonised by the International Council for Harmonisation of Technical Requirements for A platform lead by pharmaceutical specialists to grow up pharmaceutical professionals with scientific and technical knowledge
Ich Gcp Full Form

Ich Gcp Full Form
https://storage.googleapis.com/gcp-depo/demosiplah/IP/I2RMBPA07ADG1.jpg

Kurikulum Merdeka PR Bahasa Inggris Kelas VIII Semester 2 2023
https://storage.googleapis.com/gcp-depo/demosiplah/IP/I2RMBIG08B.jpg

Buku Digital Buku PR IPS Kelas VIII Semester 1 2023 SIPLah IntanOnline
https://storage.googleapis.com/gcp-depo/demosiplah/IP/I2RMBPS08ADG1.jpg
The International Council for Harmonisation of Technical Requirements of Pharmaceuticals for Human Use ICH is a unique harmonization organisation involving The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH is unique in bringing together the regulatory authorities and pharmaceutical
The ICH Harmonised Guideline was finalised under Step 4 in February 2002 This document is intended to address recommendations on the application of bracketing and matrixing to ICH brings together regulatory authorities and the pharmaceutical industry It makes recommendations towards achieving greater harmonisation in the interpretation and
More picture related to Ich Gcp Full Form

Buku Digital Buku PR PJOK Kelas VIII Semester 2 2023 SIPLah IntanOnline
https://storage.googleapis.com/gcp-depo/demosiplah/IP/I2RMBPJ08BDG.jpg

Buku Digital Buku PR Bahasa Indonesia Kelas XI Semester 2 2023
https://storage.googleapis.com/gcp-depo/demosiplah/IP/I3RMBBI11BDG.jpg

What Is The First Section Of ICH GCP Relejuvant Clinical Services
https://2.bp.blogspot.com/-_qhX3ztPYl8/WVRCu08JzyI/AAAAAAAACZA/WWwBWAcAkRUBzGaL9dRiS_KLl8i9inJXgCLcBGAs/s1600/Rel%2B23.6.17.jpg
The International Council for Harmonisation of Technical Requirements of Pharmaceuticals for Human Use ICH is a unique harmonization organisation involving regulators and the ICH has produced a comprehensive set of safety Guidelines to uncover potential risks like carcinogenicity genotoxicity and reprotoxicity A recent breakthrough has been a non clinical
[desc-10] [desc-11]

Virtual Chat On Call Dentist And 13 Core Principles Of ICH GCP Blog
http://www.virtualdentist.in/blog/wp-content/uploads/2020/08/1-2.png
The Importance Of ICH GCP CCRPS
https://images.squarespace-cdn.com/content/v1/56a042fb25981d9326c9bbdb/1620138203782-EFP6X075NA12O048FT8U/Table+1:+Reason+for+GCP

https://database.ich.org › sites › default › files
3 3 Stability Indicating Critical Quality Attributes g the principles outlined in ICH Q6A Q6B and ICH Q8 Q11 When 331 designing a stability protocol in support of a drug substance or drug

https://www.ema.europa.eu › ... › ich-guidelines
The European Medicines Agency publishes scientific guidelines on human medicines that are harmonised by the International Council for Harmonisation of Technical Requirements for

Good Clinical Practices Guideline ICH GCP Principles Of GCP Hindi

Virtual Chat On Call Dentist And 13 Core Principles Of ICH GCP Blog

CALTRANS D77B Long Beach Iron Works

Buku Digital Buku PR IPS Ekonomi Kelas XI Semester 2 2023 SIPLah

Buku Digital Buku PR PJOK Kelas XI Semester 1 2023 SIPLah IntanOnline

ICH GCP Good Clinical Practice Online Course And Certification

ICH GCP Good Clinical Practice Online Course And Certification
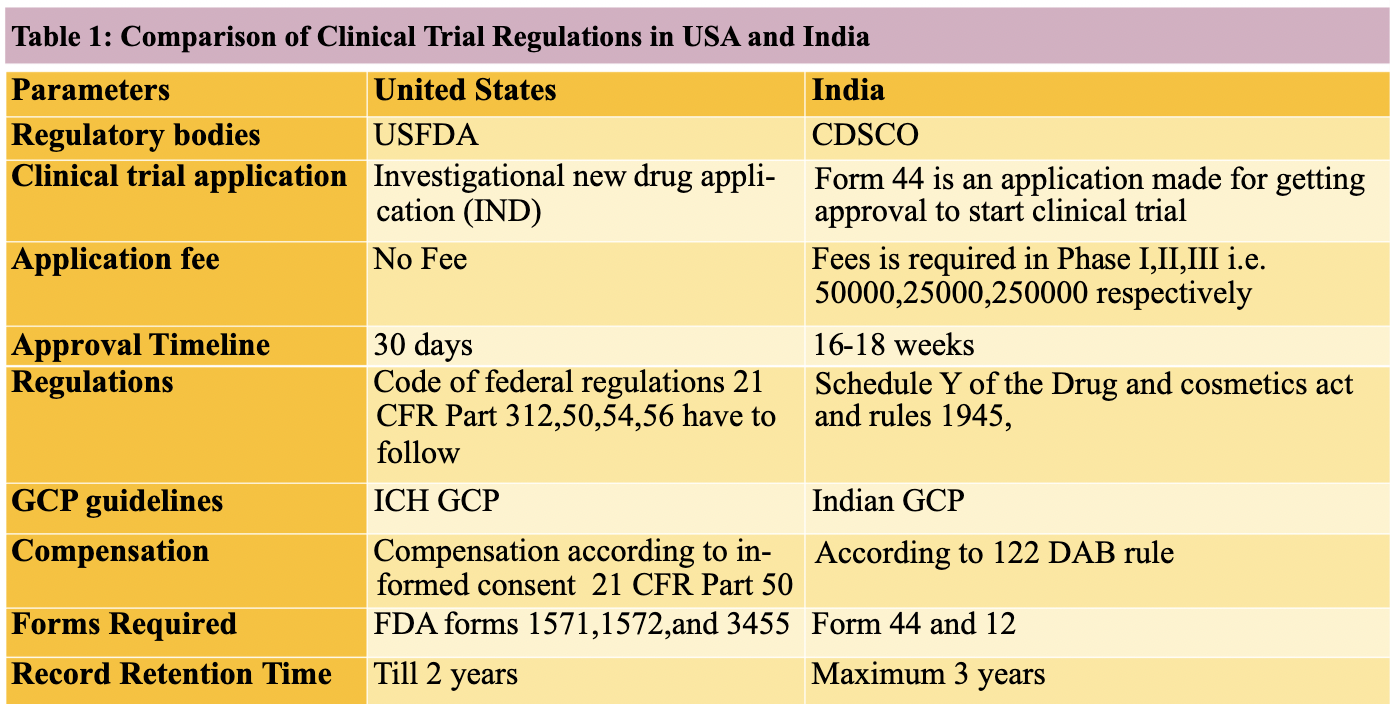
Explore Clinical Research Training Accreditation CCRPS CCRPS

13 Principles Of ICH GCP Good Clinical Practices Guidelines In

ICH GCP Training ClinAcademy Healthcare Academic Medical Institution
Ich Gcp Full Form - The ICH Harmonised Guideline was finalised under Step 4 in February 2002 This document is intended to address recommendations on the application of bracketing and matrixing to
