Epo Guidelines G1 19 EPO EPO EPO
EPO EPO 100 DOCDB INPADOC Witam mam pytanie na awizie mam przesylke listowa polecona skrot EPO czy to dotyczy wszystkich wydzialow czy tylko karne takie cos posiadaja Czy przez
Epo Guidelines G1 19

Epo Guidelines G1 19
https://www.njakers.com/wp-content/uploads/2021/05/computer-simulations-v2-1080x675.jpg

Updated EPO Guidelines 1 March 2022 Hindles
https://www.hindles.co.uk/uploads/store/mediaupload/362/image/content_1200-SgurrHeader.jpg

EPO s Article
https://www.epoartwork.com/wp-content/uploads/2023/12/epo聖誕-3-1-scaled.jpg
EPO 30 70 PE PS The fundamental principle of the Athlete Biological Passport ABP is to monitor selected biological variables over time that indirectly reveal the effects of doping rather than attempting
1 0 Introduction This Technical Document TD has been established to harmonize the detection and reporting of erythropoietin EPO and other EPO receptor agonists ERAs by WADA Technical Document TD2021EPO HARMONIZATION OF ANALYSIS AND REPORTING OF ERYTHROPOIETIN EPO AND OTHER EPO RECEPTOR AGONISTS ERAs BY
More picture related to Epo Guidelines G1 19

EPO Consultation On EPC And PCT EPO Guidelines Kluwer Patent Blog
https://wolterskluwerblogs.com/patent/wp-content/uploads/sites/52/2023/02/The_new_European_Patent_Office_in_Rijswijk-1.jpg

New EPO Guidelines For Examination 2023 Key Changes Mathys Squire LLP
https://www.mathys-squire.com/app/uploads/2023/02/shutterstock_1814564033-1-scaled.jpg

EPO New Employment Rules Roil Staff Management Responds On Demand For
https://i0.wp.com/www.ip-watch.org/weblog/wp-content/uploads/2015/06/epo-buildings-munich.jpg?ssl=1
EPO Fc 8 EPO mimetische Stoffe 8 Erythropoetine EPO 8 Erythropoetin Rezeptor Agonisten 8 Esmolol 21 Estr 4 en 3 17 diol 6 Estr 4 en 3 17 dion 6 Etacryns ure 13 Etamivan 16 The EPO Working Group provides expert advice to WADA with respect to the overall application of EPO testing including the provision of second opinions on laboratory analytical data The
[desc-10] [desc-11]

Update On ViCo Oral Proceedings At The EPO Two IP
https://www.two-ip.com/wp-content/uploads/2022/03/EPO_The-Hague_cropped-scaled.jpg

Update To The EPO Guidelines 2023 Venner Shipley
https://www.vennershipley.com/wp-content/uploads/2023/04/shutterstock_1166941312-2.jpg



Recent Updates To EPO Guidelines For Examination Novagraaf

Update On ViCo Oral Proceedings At The EPO Two IP

EPO
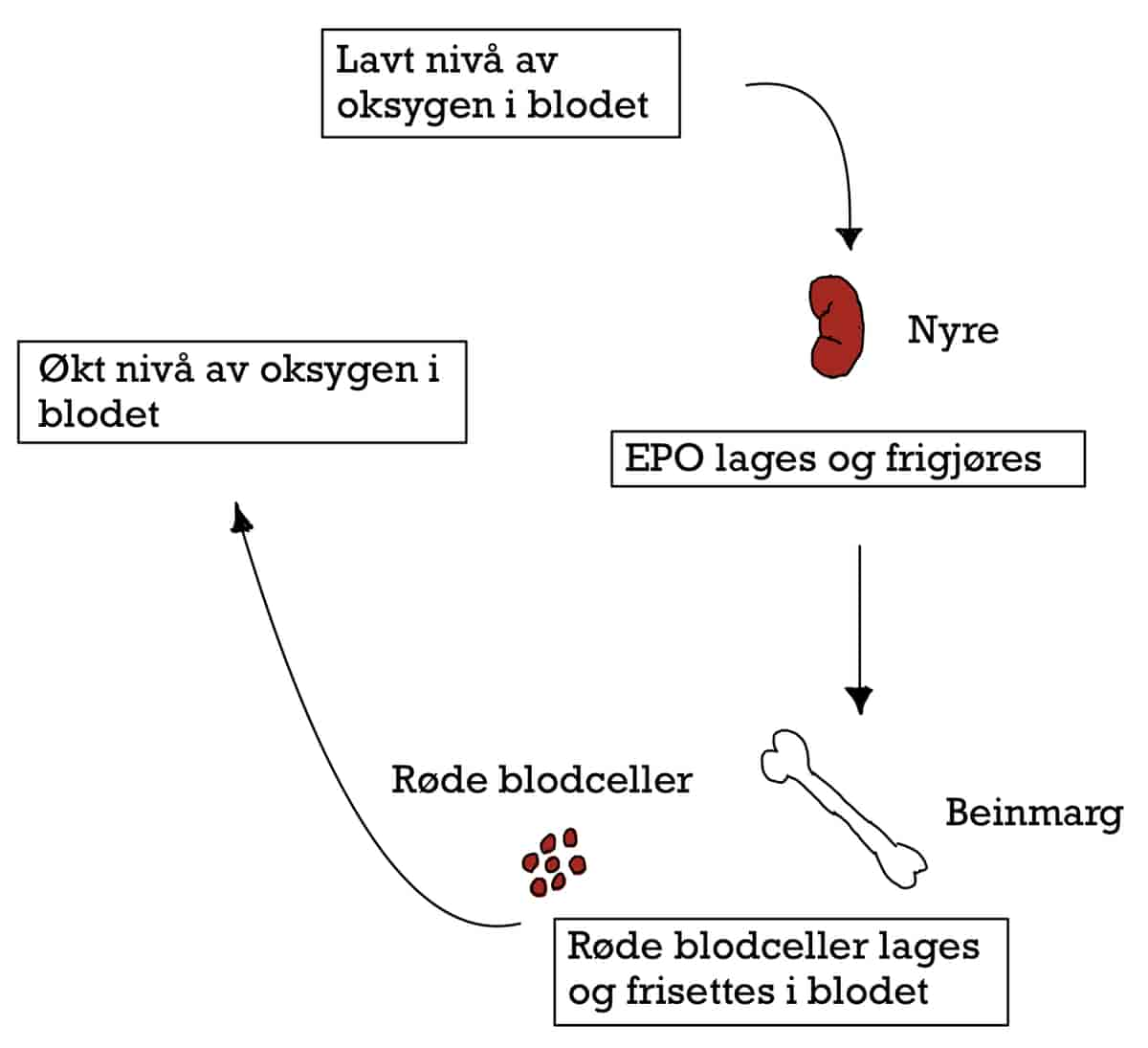
EPO Erytropoietin Store Medisinske Leksikon

Difference Between EPO And PPO Sciencerack

EPO EC Capsule 1000mg Healthcare Arogga Online Pharmacy Of Bangladesh

EPO EC Capsule 1000mg Healthcare Arogga Online Pharmacy Of Bangladesh

HY XF G1 WorkXwear

Update To The EPO Guidelines 1 March 2022 Issuu

All Software Patents Are Equal EPO Decision G1 19 Withers Rogers
Epo Guidelines G1 19 - 1 0 Introduction This Technical Document TD has been established to harmonize the detection and reporting of erythropoietin EPO and other EPO receptor agonists ERAs by