What Is The Dilution Factor Of 1 100 Dilution is the addition of solvent which decreases the concentration of the solute in the solution Concentration is the removal of solvent which increases the concentration of the solute in the
Dilution is the process of decreasing the concentration of a solute in a solution usually simply by mixing with more solvent like adding more water to the solution Dilution is a fundamental concept in chemistry that involves reducing the concentration of a substance in a solution by adding a solvent It is a technique that is used to create less
What Is The Dilution Factor Of 1 100

What Is The Dilution Factor Of 1 100
https://i.ytimg.com/vi/EOsYOfKMgS8/maxresdefault.jpg
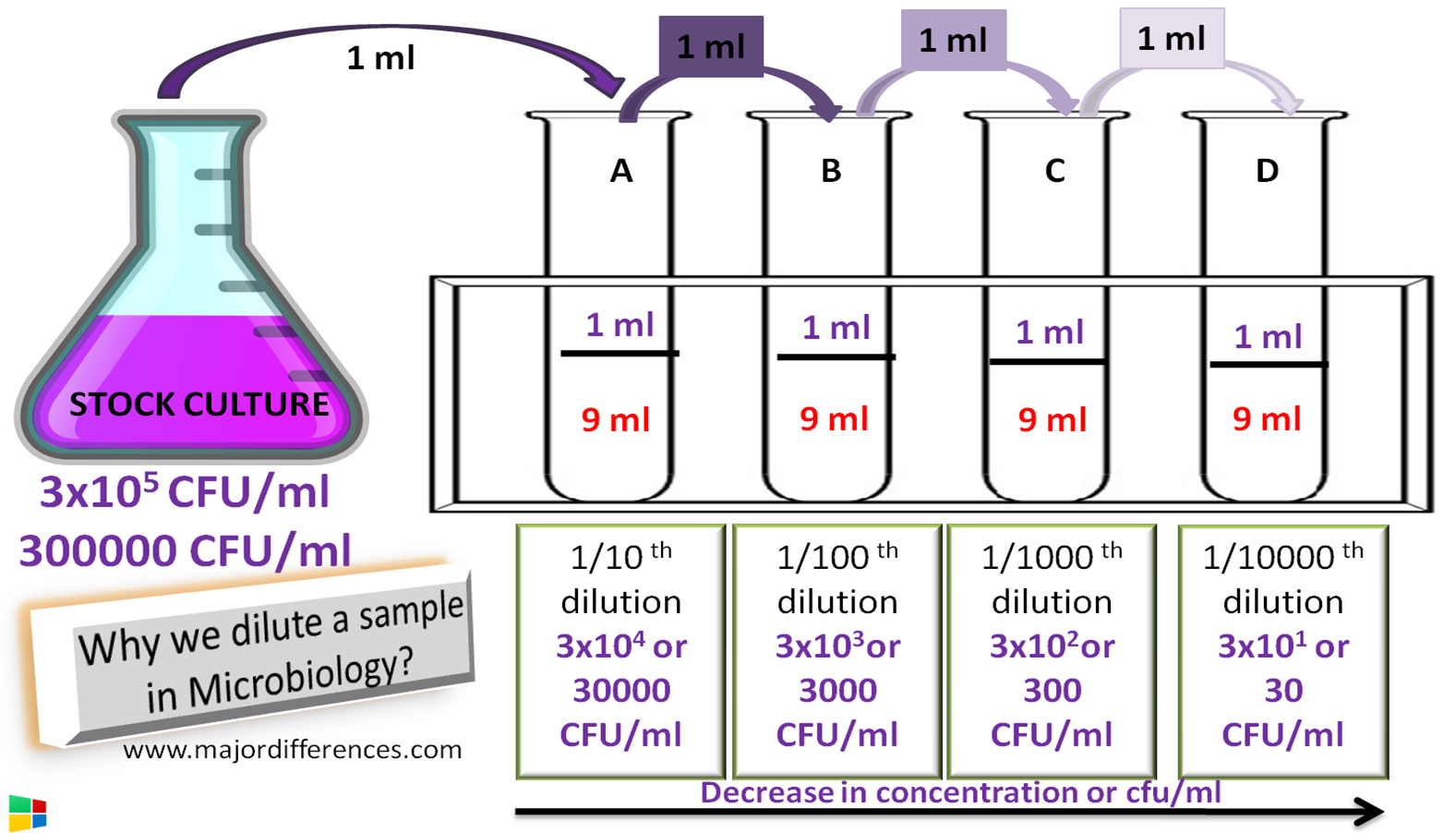
Major Differences
https://1.bp.blogspot.com/-lXUFgM-9TQE/YMhOjIoVjaI/AAAAAAAAEtU/Zl_t_UqzVTsIkgn5xu4qRiBJ_4bPUqXJgCLcBGAsYHQ/s16000/dil-1.webp
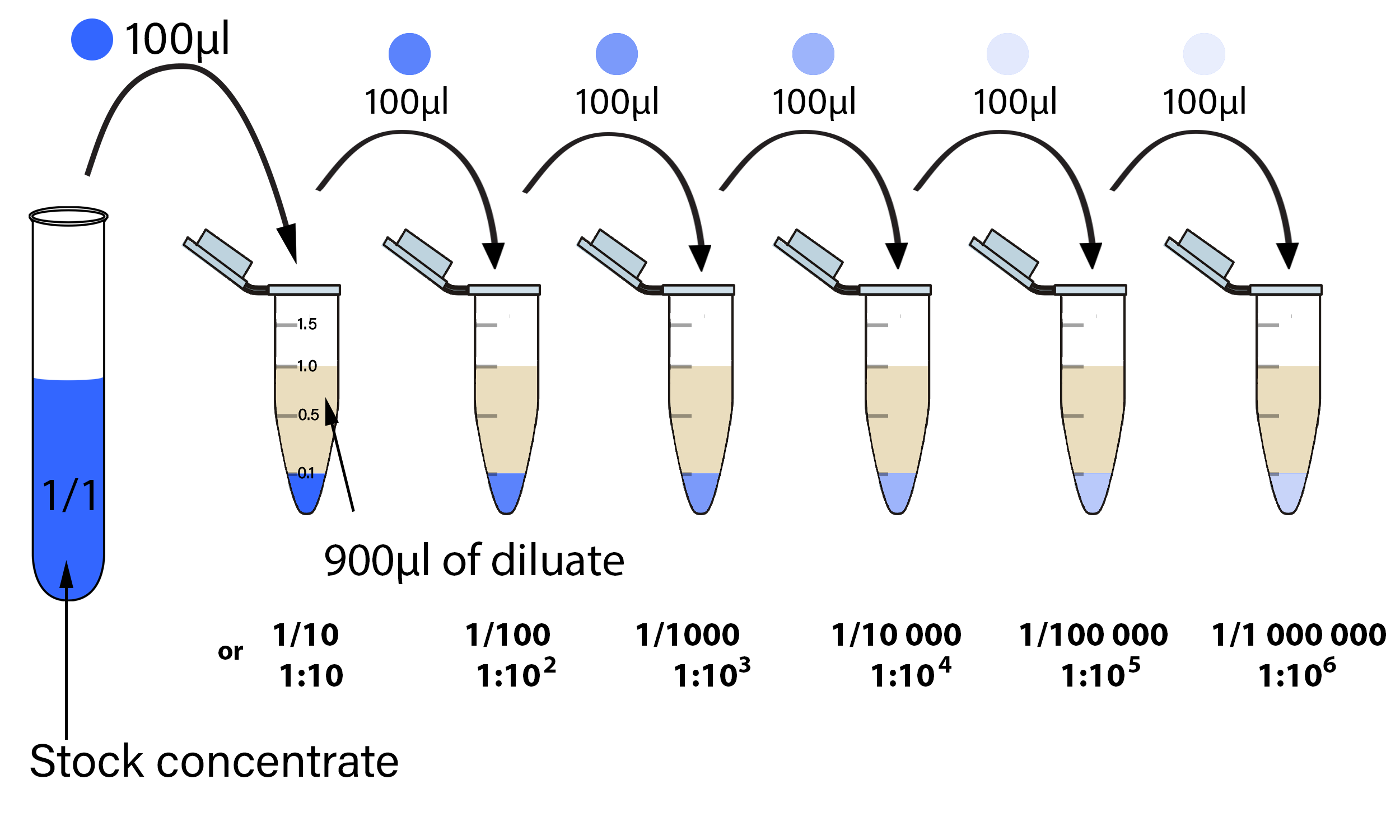
Serial Dilutions
http://www.medicine.mcgill.ca/physio/vlab/Vlab_in_progress/pics/Immunology/Serial_dilution.png
The act of reducing the concentration of a solution by adding additional solvent is known as dilution Dilution involves adding additional solvent to a sample of a solution The process does Thus we can define dilution as Dilution is the process of decreasing the concentration of a solution by adding more solvent to it Dilution may also be defined as the
Dilution is the process of decreasing the concentration of solute in a solution by changing the amount of solvent The dilute solution definition requires an understanding of This process is known as dilution because relative to the solution from which it was prepared the final solution contains the same amount of solute in a larger volume of solution and therefore
More picture related to What Is The Dilution Factor Of 1 100

Molarity Dilution Calculator Russhorn
https://i.ytimg.com/vi/JTKnmOp0I8g/maxresdefault.jpg
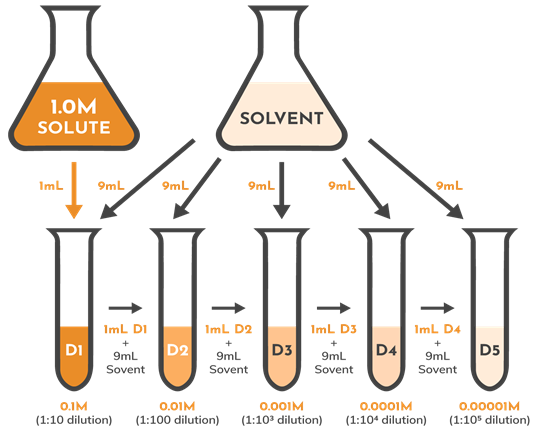
Regression Dilution Wikipedia
https://www.bosterbio.com/media/images/serial-dilution.png

Serial Dilution Technique Vrogue co
https://iul-instruments.com/wp-content/uploads/2021/01/[email protected]
Why Dilution is important Dilution of solutions helps us in many ways During titration solutions are diluted so there is a larger volume of solution available This allows the students to Another common dilution problem involves deciding how much of a highly concentrated solution is requires to make a desired quantity of solution of lesser concentration
[desc-10] [desc-11]

Dilute Science
https://img.freepik.com/premium-vector/dilution-factor-formula-science-vector-illustration-infographic_683773-1086.jpg?w=2000
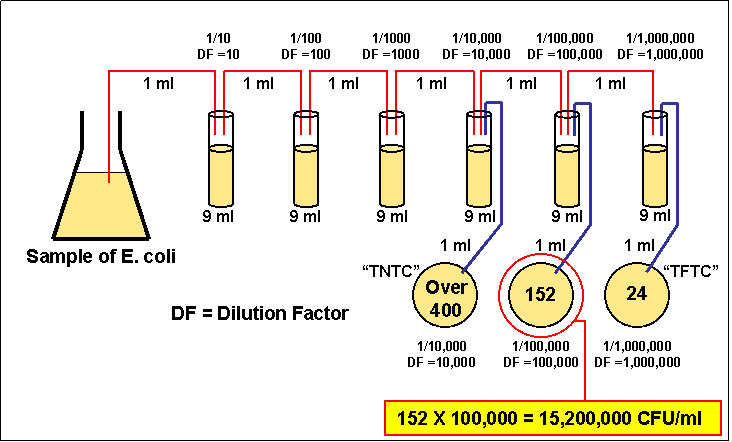
Dilution Factor Formula
http://faculty.northlandcollege.edu/biology/BioInstructors/Terry_Instruct/Microbiology/Lab 3/images/dilution series with solution.gif

https://chem.libretexts.org › Bookshelves...
Dilution is the addition of solvent which decreases the concentration of the solute in the solution Concentration is the removal of solvent which increases the concentration of the solute in the
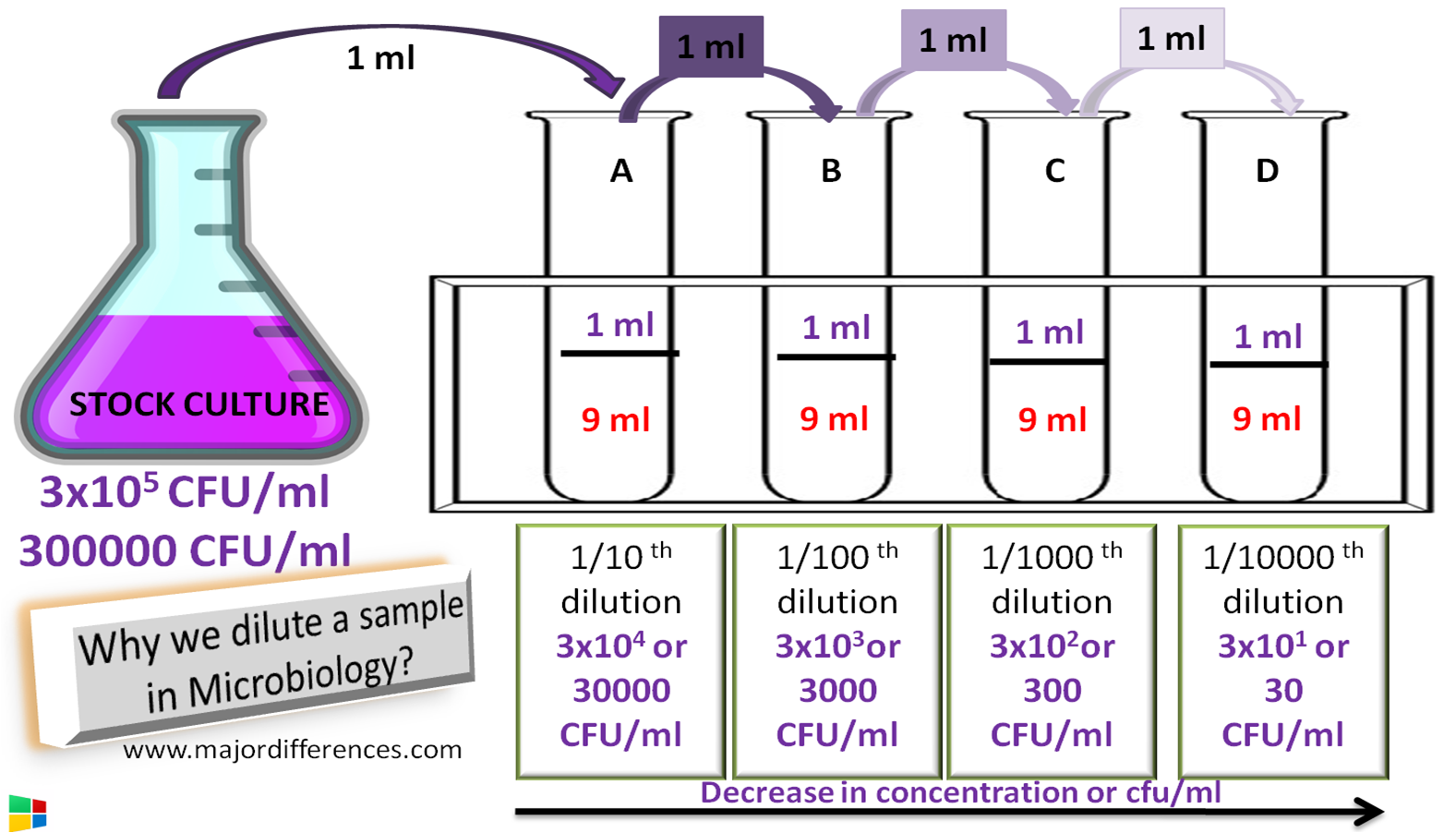
https://en.wikipedia.org › wiki › Dilution_(equation)
Dilution is the process of decreasing the concentration of a solute in a solution usually simply by mixing with more solvent like adding more water to the solution

Serial Dilution Formula Calculator Method Uses Examples

Dilute Science
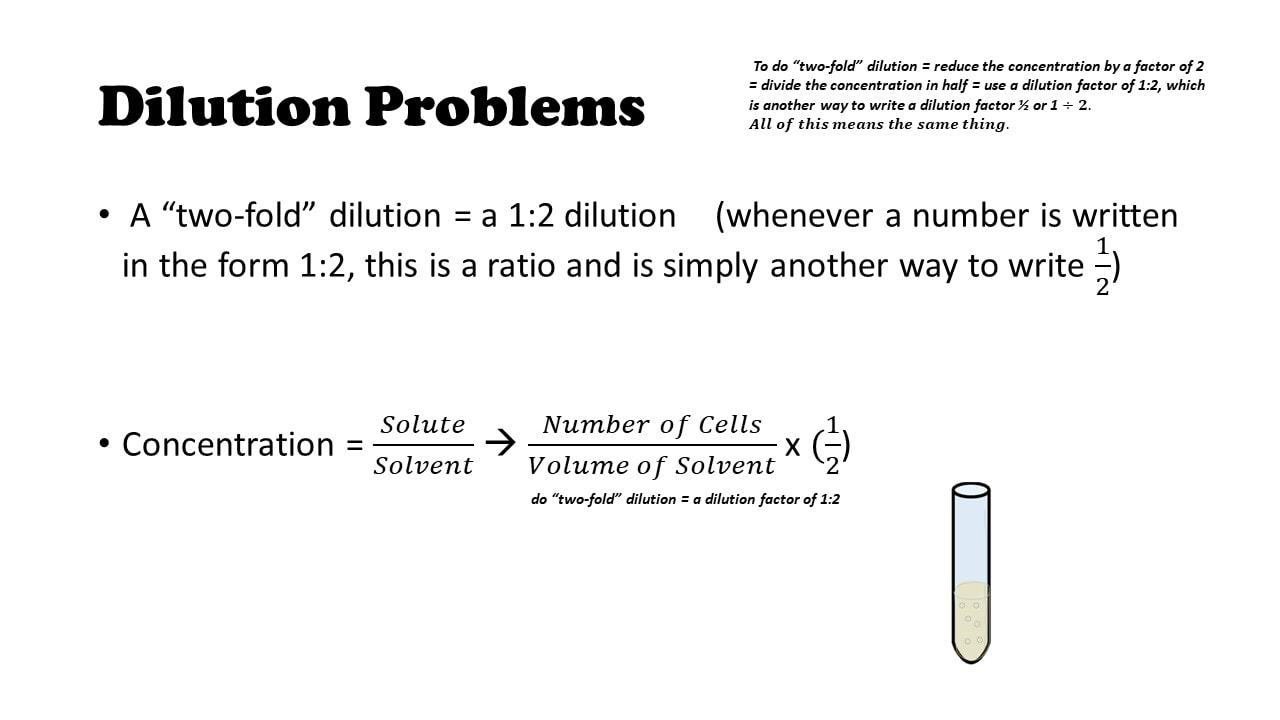
Dilution Calculations Worksheet
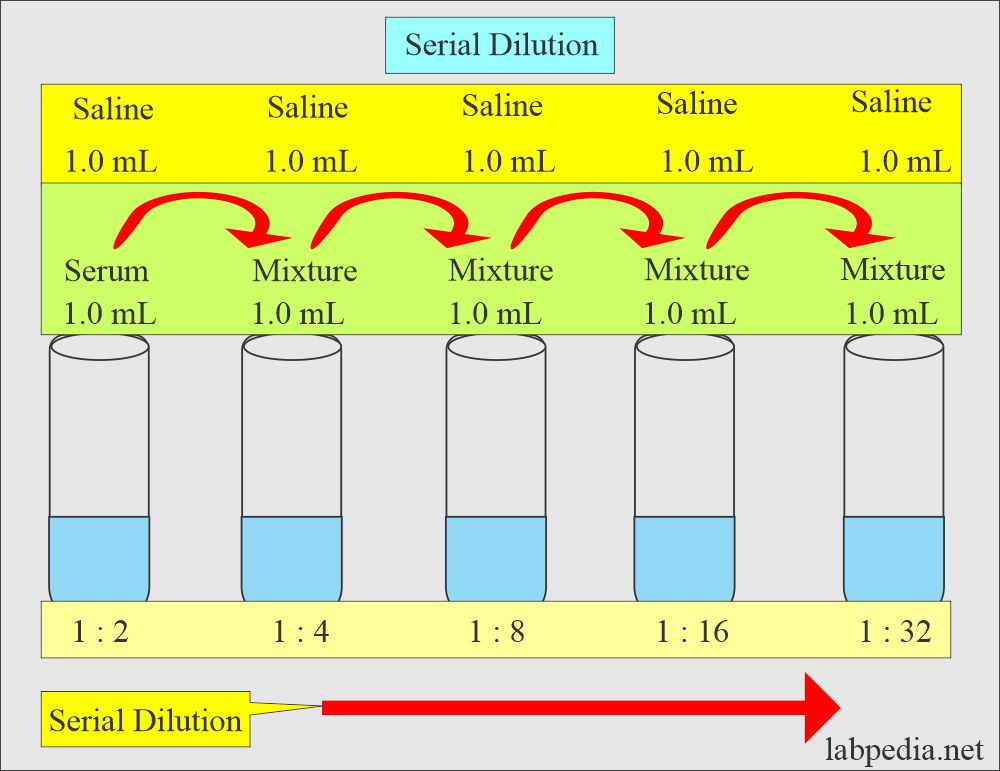
Solutions Part 3 Solutions With Various Examples Labpedia

Chemical Dilution Rate Guide Dalcon Hygiene

Calculate Dilution Startup

Calculate Dilution Startup

10 Fold Serial Dilution

Serial Dilution Diagram

1 10 Dilution Of Bleach
What Is The Dilution Factor Of 1 100 - [desc-12]